
Background
Several agents have been investigated for beyond second-line treatment of chronic graft-versus-host disease (GVHD). Ruxolitinib has been recently approved for steroid-refractory acute GVHD, while a prospective randomized study is ongoing to examine its efficacy in steroid-resistant chronic GVHD (cGVHD). The present study evaluated the efficacy of Ruxolitinib in terms of 1) overall response rate (ORR), 2) clinical benefit (CB), 3) dose reduction of corticosteroid exposure, 4) failure-free survival (FFS) and 5) overall survival (OS), in patients heavily pretreated for steroid-resistant cGVHD.
Patients and methods
A total of 47 patients who developed cGVHD after HCT and treated with Ruxolitinib for cGVHD from March 2016 to April 2020, at three different sites (Princess Margaret Cancer Center, Canada; Vancouver General Hospital, Canada and Saint Vincent Hospital, Australia), were evaluated in the retrospective study. Patients and disease characteristics are as follows: median age 52 years; classical 35 (71%), overlap syndrome 14 (29%). Of note, 27 patients (57.4%) had a previous history of acute GVHD.
The ORR and CB were assessed at months 3, 6 and 12, retrospectively. Responses were evaluated according to NIH scoring/staging/response assessment as part of standard clinical practice. CB was assessed considering clinical response as well as steroid dose reduction. For systemic steroid dose reduction, prednisone dose per kg per day was captured prior to Ruxolitinib start, at months 3, 6 and 12. Treatment failure was defined as 1) resistance requiring treatment switch, 2) non-relapse mortality (NRM), 3) relapse, 4) intolerance to treatment. FFS and OS were calculated from the day of starting Ruxolitinib therapy for cGVHD treatment.
Results
A total of 47 patients had moderate (11/47, 24.4%) to severe (33/47, 73.3%) cGVHD except one who had mild grade cGVHD with a high-risk feature (thrombocytopenia at the time of Ruxolitinib start). The median number of organ involvement was 3 (range 1-7). Over half of patients (63.8%) received Ruxolitinib as 4th line or beyond for cGVHD treatment, while median number of previous lines of therapy was 3 (range 1-9). All 47 patients (100%) had been previously treated with systemic steroids; other previous treatments included ECP therapy (53.2%), Imatinib (29.8%), Ibrutinib (23.4%), Rituximab (21.3%).
Ruxolitinib was started at 10-15 mg daily as initial dose, then maintained at 20mg daily in two divided doses on months 3, 6 and 12.
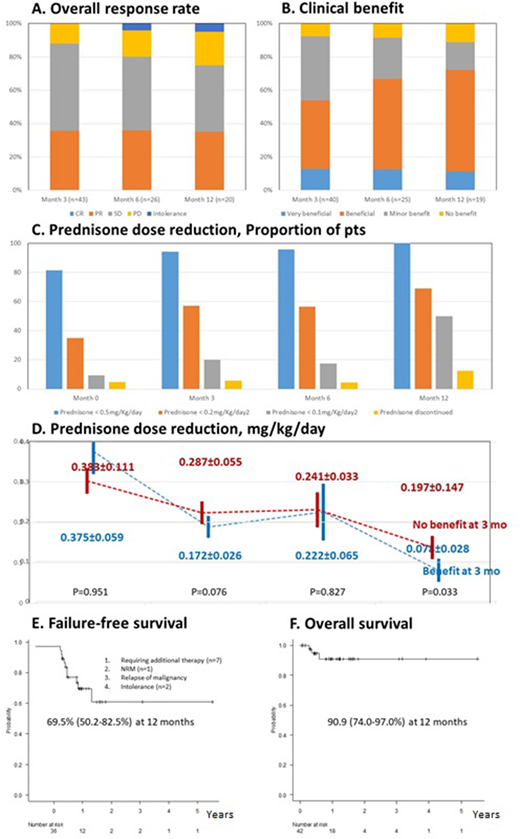
With a median follow-up duration of 12 months, ORR was attained in 35.7%, 36.0% and 35.0% at 3, 6 and 12 months, respectively (Figure A). Of note, ORR in patients with sclerotic changes was 56%, and 61.5% in those with lung involvement. Patients resistant to TKI (i.e. Imatinib or Ibrutinib) for cGVHD treatment showed similar ORR compared to those naïve to TKI therapy.
The CB was observed in 53.5%, 66.7% and 72.2% at months 3, 6 and 12, respectively (Figure B). Patients resistant to TKI for cGVHD treatment did not show any difference in CB compared to those naïve to TKI therapy.
In terms of prednisone dose reduction, by 12 months , half of patients (50.0%) could taper prednisone doses below 0.1mg/kg/day, while the proportion of patients on prednisone dose below 0.1mg/kg/day was 9.3%, 20.0%, 17.4%, and 50.0% at month 0, 3, 6 and 12, respectively (Figure C). The group who achieved CB at 3 months showed a significantly lower dose of prednisone at 12 months (0.078mg/kg/day) compared to those without clinical benefit at 3 months (0.197mg/kg/day; p=0.033; Figure D).
Out of 37 patients evaluated, 11 failures (29.7%) were noted, including resistance requiring a switch to other therapy (n=7), NRM (n=2) and intolerance (n=2). The FFS rate at 1 year in the overall population was 68.5% (Figure E). The FFS at 1 year in those having CB at 3 months vs not was 86.5% vs 51.4% (p=0.025).
The OS at 1 year was 90.9% (Figure F). The OS at 1 year in those having a CB at 3 months vs not was 100% vs 78.8% (p=0.053).
Conclusion:
This multicenter retrospective study revealed that Ruxolitinib is an effective treatment option for patients with cGVHD, with good ORR and CB. The achievement of CB in the first 3 months correlated well with steroid dose reduction. It suggests that Ruxolitinib is a feasible GVHD treatment option, even for patients who were heavily pretreated for cGVHD or failed previous TKI drug.
Hamad:Abbvie: Honoraria; Novartis: Honoraria.
Ruxolitinib treatment for steroid resistant chronic GVHD
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal